News
The Shanghai Canbi news column provides information on reference preparations, pharmaceutical intermediates, development of pharmaceutical raw materials and blood products in the field of biomedicine. Access to the latest and most comprehensive medical news policies through various global medical news platforms, collect the latest medical research and development data, take you to understand the latest trends and policies of the pharmaceutical industry, stay at home to read the latest global medical news and information.
-
 UBIX THERAPEUTICS KICK OFF AN IND-ENABLING STUDIS OF BRUTON’S TYROSINE KINASE DEGRADERSeoul, Republic of Korea – July 20th, 2021 – South Korea-based biotech company, Ubix Therapeutics (en.ubixtrx.com), announced the current status of their lead program, UBX-303, which utilizes Ubix’s platform technology, Degraducer®. UBX-303 is a BrTags:The number of readers:1851Source:2021-07-20
UBIX THERAPEUTICS KICK OFF AN IND-ENABLING STUDIS OF BRUTON’S TYROSINE KINASE DEGRADERSeoul, Republic of Korea – July 20th, 2021 – South Korea-based biotech company, Ubix Therapeutics (en.ubixtrx.com), announced the current status of their lead program, UBX-303, which utilizes Ubix’s platform technology, Degraducer®. UBX-303 is a BrTags:The number of readers:1851Source:2021-07-20 -
 US FDA approves fexinidazole as the first all-oral treatment for sleeping sicknessThe US Food and Drug Administration (FDA) has approved fexinidazole as the first all-oral treatment for both stages of the Trypanosoma brucei gambiense form of sleeping sickness (Human African trypanosomiasis) in patients 6 years of age and older and weigTags:The number of readers:1878Source:2021-07-19
US FDA approves fexinidazole as the first all-oral treatment for sleeping sicknessThe US Food and Drug Administration (FDA) has approved fexinidazole as the first all-oral treatment for both stages of the Trypanosoma brucei gambiense form of sleeping sickness (Human African trypanosomiasis) in patients 6 years of age and older and weigTags:The number of readers:1878Source:2021-07-19 -
 KEYTRUDA®(pembrolizumab) Plus Chemotherapy Before Surgery and Continued as a Single Agent After Surgery Showed Statistically Significant Event-Free Survival (EFS) Result Versus NeoadjuMerck (NYSE: MRK), known as MSD outside the United States and Canada, today announced positive event-free survival (EFS) data from the pivotal neoadjuvant/adjuvant Phase 3 study KEYNOTE-522. The trial investigated neoadjuvant KEYTRUDA, Merck’s anti-PD-1Tags:The number of readers:1539Source:2021-07-16
KEYTRUDA®(pembrolizumab) Plus Chemotherapy Before Surgery and Continued as a Single Agent After Surgery Showed Statistically Significant Event-Free Survival (EFS) Result Versus NeoadjuMerck (NYSE: MRK), known as MSD outside the United States and Canada, today announced positive event-free survival (EFS) data from the pivotal neoadjuvant/adjuvant Phase 3 study KEYNOTE-522. The trial investigated neoadjuvant KEYTRUDA, Merck’s anti-PD-1Tags:The number of readers:1539Source:2021-07-16 -
 Incyte Announces Positive Phase 3 REACH3 Study Data Published in NEJM for Ruxolitinib (Jakafi®) in Chronic Graft-Versus-Host DiseaseIncyte (Nasdaq:INCY) today announced that positive data from the Phase 3 REACH3 study have been published in The New England Journal of Medicine (NEJM) demonstrating that treatment with ruxolitinib (Jakafi®) resulted in significantly improved outcomes inTags:The number of readers:1469Source:2021-07-15
Incyte Announces Positive Phase 3 REACH3 Study Data Published in NEJM for Ruxolitinib (Jakafi®) in Chronic Graft-Versus-Host DiseaseIncyte (Nasdaq:INCY) today announced that positive data from the Phase 3 REACH3 study have been published in The New England Journal of Medicine (NEJM) demonstrating that treatment with ruxolitinib (Jakafi®) resulted in significantly improved outcomes inTags:The number of readers:1469Source:2021-07-15 -
 Kintor Pharmaceutical Receives IND Clearance by the U.S. FDA for GT20029 to Treat Androgenetic Alopecia and AcneSuzhou Kintor Pharma Limited (HKEX:9939), a clinical-stage biotechnology company developing innovative small-molecule and biological therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has cleared an Investigational New Drug (INTags:The number of readers:1859Source:2021-07-14
Kintor Pharmaceutical Receives IND Clearance by the U.S. FDA for GT20029 to Treat Androgenetic Alopecia and AcneSuzhou Kintor Pharma Limited (HKEX:9939), a clinical-stage biotechnology company developing innovative small-molecule and biological therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has cleared an Investigational New Drug (INTags:The number of readers:1859Source:2021-07-14 -
 CD47 Monoclonal Antibody (AK117) Completed Phase I Dose Escalation Trial and Obtained Approval to Initiate Clinical Trial in Combination with Azacitidine for Treatment of Acute Myeloid Leukemiakeso Biopharma Inc.. is pleased to announce that, CD47 monoclonal antibody (AK117), a second-generation novel drug for immuno-oncology therapy independently developed by the Company, has completed phase I dose escalation trial in Australia. AK117 resultedTags:The number of readers:1920Source:2021-07-13
CD47 Monoclonal Antibody (AK117) Completed Phase I Dose Escalation Trial and Obtained Approval to Initiate Clinical Trial in Combination with Azacitidine for Treatment of Acute Myeloid Leukemiakeso Biopharma Inc.. is pleased to announce that, CD47 monoclonal antibody (AK117), a second-generation novel drug for immuno-oncology therapy independently developed by the Company, has completed phase I dose escalation trial in Australia. AK117 resultedTags:The number of readers:1920Source:2021-07-13 -
 U.S. FDA Grants Regular Approval and Expands Indication for PADCEV®(enfortumab vedotin-ejfv) for Patients with Locally Advanced or Metastatic Urothelial CancerSeagen Inc. (Nasdaq:SGEN) and Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., “Astellas”) today announced the U.S. Food and Drug Administration (FDA) granted PADCEV® (enfortumab vedotin-ejfv) regular approval in the U.S., iTags:The number of readers:1806Source:2021-07-12
U.S. FDA Grants Regular Approval and Expands Indication for PADCEV®(enfortumab vedotin-ejfv) for Patients with Locally Advanced or Metastatic Urothelial CancerSeagen Inc. (Nasdaq:SGEN) and Astellas Pharma Inc. (TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., “Astellas”) today announced the U.S. Food and Drug Administration (FDA) granted PADCEV® (enfortumab vedotin-ejfv) regular approval in the U.S., iTags:The number of readers:1806Source:2021-07-12 -
 ASC Therapeutics Receives IND Clearance From the U.S. Food and Drug Administration for ASC618 Second-Generation Gene Therapy for Hemophilia AASC Therapeutics, a privately-held biopharmaceutical company pioneering the development of transformative in-vivo gene replacement, gene editing and allogeneic cell therapies for hematologic and other rare disorders today announced that the U.S. Food & DrTags:The number of readers:1598Source:2021-07-08
ASC Therapeutics Receives IND Clearance From the U.S. Food and Drug Administration for ASC618 Second-Generation Gene Therapy for Hemophilia AASC Therapeutics, a privately-held biopharmaceutical company pioneering the development of transformative in-vivo gene replacement, gene editing and allogeneic cell therapies for hematologic and other rare disorders today announced that the U.S. Food & DrTags:The number of readers:1598Source:2021-07-08 -
 EpimAb Biotherapeutics Provides Clinical Update for EMB-02 and EMB-06EpimAb Biotherapeutics, a clinical stage biotech company specializing in bispecific antibodies, today announced dose administration for the first patient in a Phase 1/2 clinical trial of EMB-02, a bispecific antibody targeting PD-1 and LAG-3, in patientsTags:The number of readers:1747Source:2021-07-07
EpimAb Biotherapeutics Provides Clinical Update for EMB-02 and EMB-06EpimAb Biotherapeutics, a clinical stage biotech company specializing in bispecific antibodies, today announced dose administration for the first patient in a Phase 1/2 clinical trial of EMB-02, a bispecific antibody targeting PD-1 and LAG-3, in patientsTags:The number of readers:1747Source:2021-07-07 -
 GenSight Biologics Announces Approval of the LUMEVOQ® Cohort Temporary Authorization for Use (ATUc) in FranceGenSight Biologics (Paris:SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders,Tags:The number of readers:1717Source:2021-07-05
GenSight Biologics Announces Approval of the LUMEVOQ® Cohort Temporary Authorization for Use (ATUc) in FranceGenSight Biologics (Paris:SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders,Tags:The number of readers:1717Source:2021-07-05

Featured Products
-
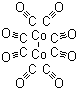 Dicobalt octacarbonylCAS:10210-68-1Molecular Formula:Co2(CO)8
Dicobalt octacarbonylCAS:10210-68-1Molecular Formula:Co2(CO)8 -
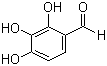 2,3,4-TrihydroxybenzaldehydeCAS:2144-08-3Molecular Formula:C7H6O4
2,3,4-TrihydroxybenzaldehydeCAS:2144-08-3Molecular Formula:C7H6O4 -
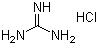 Guanidine hydrochlorideCAS:50-01-1Molecular Formula:CH5N3.HCl
Guanidine hydrochlorideCAS:50-01-1Molecular Formula:CH5N3.HCl -
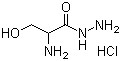 DL-Serinohydrazide monohydrochlorideCAS:55819-71-1Molecular Formula:C3H9N3O2.ClH
DL-Serinohydrazide monohydrochlorideCAS:55819-71-1Molecular Formula:C3H9N3O2.ClH -
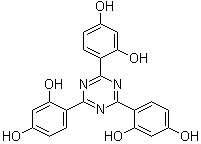 2,4,6-Tri(2,4-dihydroxyphenyl)-1,3,5-triazineCAS:2125-23-7Molecular Formula:C21H15N3O6
2,4,6-Tri(2,4-dihydroxyphenyl)-1,3,5-triazineCAS:2125-23-7Molecular Formula:C21H15N3O6 -
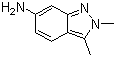 2,3-Dimethyl-6-amino-2H-indazoleCAS:444731-72-0Molecular Formula:C9H11N3
2,3-Dimethyl-6-amino-2H-indazoleCAS:444731-72-0Molecular Formula:C9H11N3 -
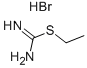 S-ETHYLISOTHIOUREA HYDROBROMIDECAS:1071-37-0Molecular Formula:
S-ETHYLISOTHIOUREA HYDROBROMIDECAS:1071-37-0Molecular Formula: -
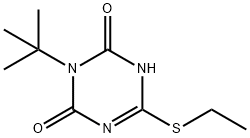 1,3,5-Triazine-2,4(1H,3H)-dione, 3-(1,1-dimethylethyl)-6-(ethylthio)-CAS:1360105-53-8Molecular Formula:
1,3,5-Triazine-2,4(1H,3H)-dione, 3-(1,1-dimethylethyl)-6-(ethylthio)-CAS:1360105-53-8Molecular Formula:
Hot Tags
Inquiry
Fill in related informations ;please!
- Please fill in product name !
- CAS
- Please fill in your company information correctly!
- Please fill in liaison information correctly!
- Please fill in your telephone number correctly!
- Please fill in your email correctly!
- Please describe the problem of products!
Latest Q&A


