News
The Shanghai Canbi news column provides information on reference preparations, pharmaceutical intermediates, development of pharmaceutical raw materials and blood products in the field of biomedicine. Access to the latest and most comprehensive medical news policies through various global medical news platforms, collect the latest medical research and development data, take you to understand the latest trends and policies of the pharmaceutical industry, stay at home to read the latest global medical news and information.
-
 Gilead Submits New Drug Application to U.S. Food and Drug Administration for Lenacapavir, an Investigational, Long-Acting Capsid Inhibitor for the Treatment of HIV-1 in People With Limited TherapyGilead Sciences, Inc. (Nasdaq: GILD) announced today that the company completed submission of a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking approval of lenacapavir, an investigational, long-acting HIV-1 capsid inhibitTags:The number of readers:1563Source:2021-06-29
Gilead Submits New Drug Application to U.S. Food and Drug Administration for Lenacapavir, an Investigational, Long-Acting Capsid Inhibitor for the Treatment of HIV-1 in People With Limited TherapyGilead Sciences, Inc. (Nasdaq: GILD) announced today that the company completed submission of a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking approval of lenacapavir, an investigational, long-acting HIV-1 capsid inhibitTags:The number of readers:1563Source:2021-06-29 -
 Sandoz announces EU launch of ready-to-dilute generic Pemetrexed to treat most prevalent form of lung cancerSandoz today announced the launch of generic oncology treatment Pemetrexed in 11 countries across Europe, including Germany, Switzerland, Netherlands, and Spain.Tags:The number of readers:1569Source:2021-06-25
Sandoz announces EU launch of ready-to-dilute generic Pemetrexed to treat most prevalent form of lung cancerSandoz today announced the launch of generic oncology treatment Pemetrexed in 11 countries across Europe, including Germany, Switzerland, Netherlands, and Spain.Tags:The number of readers:1569Source:2021-06-25 -
 FDA Accepts Application for Genentech’s Port Delivery System With Ranibizumab (PDS) for Treatment of Wet Age-Related Macular Degeneration (AMD)Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s Biologics License Application (BLA), under Priority Review, for Port Delivery System with raTags:The number of readers:1614Source:2021-06-24
FDA Accepts Application for Genentech’s Port Delivery System With Ranibizumab (PDS) for Treatment of Wet Age-Related Macular Degeneration (AMD)Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s Biologics License Application (BLA), under Priority Review, for Port Delivery System with raTags:The number of readers:1614Source:2021-06-24 -
 China NMPA Approves Tislelizumab in Non-Small Cell Lung Cancer and Hepatocellular CarcinomaBeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160), a global biotechnology company focused on developing and commercializing innovative medicines worldwide, today announced that the China National Medical Products Administration (NMPA) has granted its anti-PD-1 anTags:The number of readers:1545Source:2021-06-23
China NMPA Approves Tislelizumab in Non-Small Cell Lung Cancer and Hepatocellular CarcinomaBeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160), a global biotechnology company focused on developing and commercializing innovative medicines worldwide, today announced that the China National Medical Products Administration (NMPA) has granted its anti-PD-1 anTags:The number of readers:1545Source:2021-06-23 -
 FDA Approves Pradaxa (dabigatran) as First Oral Blood Thinning Medication for ChildrenToday, the U.S. Food and Drug Administration approved Pradaxa (dabigatran etexilate) oral pellets to treat children 3 months to less than 12 years old with venous thromboembolism (a condition where blood clots form in the veins) directly after they have bTags:The number of readers:1507Source:2021-06-22
FDA Approves Pradaxa (dabigatran) as First Oral Blood Thinning Medication for ChildrenToday, the U.S. Food and Drug Administration approved Pradaxa (dabigatran etexilate) oral pellets to treat children 3 months to less than 12 years old with venous thromboembolism (a condition where blood clots form in the veins) directly after they have bTags:The number of readers:1507Source:2021-06-22 -
 Health Canada Grants Marketing Authorization for TRIKAFTA® in People Ages 12 and Older Who Have at Least One F508del MutationVertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced Health Canada has granted Marketing Authorization for TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor) for the treatment of cystic fibrosis (CF) in people ages 12 years and oldeTags:The number of readers:1532Source:2021-06-19
Health Canada Grants Marketing Authorization for TRIKAFTA® in People Ages 12 and Older Who Have at Least One F508del MutationVertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced Health Canada has granted Marketing Authorization for TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor) for the treatment of cystic fibrosis (CF) in people ages 12 years and oldeTags:The number of readers:1532Source:2021-06-19 -
 FDA Approves Astepro® Allergy Nasal Spray for Over-the-Counter Use in the United StatesBayer announced today that the U.S. Food and Drug Administration (FDA) has approved Astepro® Allergy (Azelastine HCI .15%) as an over-the-counter (OTC) product for the temporary relief of nasal congestion, runny nose, sneezing and itchy nose due to hay fTags:The number of readers:1540Source:2021-06-18
FDA Approves Astepro® Allergy Nasal Spray for Over-the-Counter Use in the United StatesBayer announced today that the U.S. Food and Drug Administration (FDA) has approved Astepro® Allergy (Azelastine HCI .15%) as an over-the-counter (OTC) product for the temporary relief of nasal congestion, runny nose, sneezing and itchy nose due to hay fTags:The number of readers:1540Source:2021-06-18 -
 FDA Approves Rezipres (ephedrine hydrochloride injection) for Hypotension During AnesthesiaEton Pharmaceuticals, Inc today announced that the U.S. Food and Drug Administration (FDA) has approved Rezipres (ephedrine hydrochloride injection) for the treatment of clinically important hypotension occurring in the setting of anesthesia.Tags:The number of readers:1525Source:2021-06-16
FDA Approves Rezipres (ephedrine hydrochloride injection) for Hypotension During AnesthesiaEton Pharmaceuticals, Inc today announced that the U.S. Food and Drug Administration (FDA) has approved Rezipres (ephedrine hydrochloride injection) for the treatment of clinically important hypotension occurring in the setting of anesthesia.Tags:The number of readers:1525Source:2021-06-16 -
 New Roche data for Evrysdi show improved motor function in pre-symptomatic babies after one year and confirm safety profile in previously treated people with spinal muscular atrophy (SMA)Roche today announced new interim data from two studies of Evrysdi® (risdiplam); JEWELFISH and RAINBOWFISH. Data from JEWELFISH, an ongoing open-label study primarily evaluating the safety of Evrysdi in people aged 1 to 60 years who have been previouslyTags:The number of readers:1477Source:2021-06-11
New Roche data for Evrysdi show improved motor function in pre-symptomatic babies after one year and confirm safety profile in previously treated people with spinal muscular atrophy (SMA)Roche today announced new interim data from two studies of Evrysdi® (risdiplam); JEWELFISH and RAINBOWFISH. Data from JEWELFISH, an ongoing open-label study primarily evaluating the safety of Evrysdi in people aged 1 to 60 years who have been previouslyTags:The number of readers:1477Source:2021-06-11 -
 Lyra Therapeutics Announces Positive Outcome of End-of-Phase 2 Meeting with the FDA for LYR-210 for the Treatment of Chronic RhinosinusitisLyra Therapeutics, Inc. (Nasdaq: LYRA), a clinical-stage therapeutics company leveraging its proprietary XTreo™ platform to enable precise, sustained, and local delivery of medications to the ear, nose and throat (ENT) passages and other diseased tissueTags:The number of readers:1483Source:2021-06-09
Lyra Therapeutics Announces Positive Outcome of End-of-Phase 2 Meeting with the FDA for LYR-210 for the Treatment of Chronic RhinosinusitisLyra Therapeutics, Inc. (Nasdaq: LYRA), a clinical-stage therapeutics company leveraging its proprietary XTreo™ platform to enable precise, sustained, and local delivery of medications to the ear, nose and throat (ENT) passages and other diseased tissueTags:The number of readers:1483Source:2021-06-09

Featured Products
-
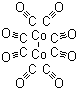 Dicobalt octacarbonylCAS:10210-68-1Molecular Formula:Co2(CO)8
Dicobalt octacarbonylCAS:10210-68-1Molecular Formula:Co2(CO)8 -
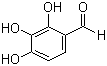 2,3,4-TrihydroxybenzaldehydeCAS:2144-08-3Molecular Formula:C7H6O4
2,3,4-TrihydroxybenzaldehydeCAS:2144-08-3Molecular Formula:C7H6O4 -
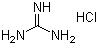 Guanidine hydrochlorideCAS:50-01-1Molecular Formula:CH5N3.HCl
Guanidine hydrochlorideCAS:50-01-1Molecular Formula:CH5N3.HCl -
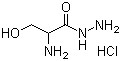 DL-Serinohydrazide monohydrochlorideCAS:55819-71-1Molecular Formula:C3H9N3O2.ClH
DL-Serinohydrazide monohydrochlorideCAS:55819-71-1Molecular Formula:C3H9N3O2.ClH -
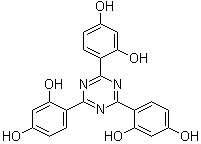 2,4,6-Tri(2,4-dihydroxyphenyl)-1,3,5-triazineCAS:2125-23-7Molecular Formula:C21H15N3O6
2,4,6-Tri(2,4-dihydroxyphenyl)-1,3,5-triazineCAS:2125-23-7Molecular Formula:C21H15N3O6 -
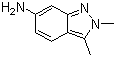 2,3-Dimethyl-6-amino-2H-indazoleCAS:444731-72-0Molecular Formula:C9H11N3
2,3-Dimethyl-6-amino-2H-indazoleCAS:444731-72-0Molecular Formula:C9H11N3 -
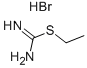 S-ETHYLISOTHIOUREA HYDROBROMIDECAS:1071-37-0Molecular Formula:
S-ETHYLISOTHIOUREA HYDROBROMIDECAS:1071-37-0Molecular Formula: -
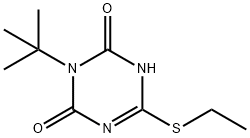 1,3,5-Triazine-2,4(1H,3H)-dione, 3-(1,1-dimethylethyl)-6-(ethylthio)-CAS:1360105-53-8Molecular Formula:
1,3,5-Triazine-2,4(1H,3H)-dione, 3-(1,1-dimethylethyl)-6-(ethylthio)-CAS:1360105-53-8Molecular Formula:
Hot Tags
Inquiry
Fill in related informations ;please!
- Please fill in product name !
- CAS
- Please fill in your company information correctly!
- Please fill in liaison information correctly!
- Please fill in your telephone number correctly!
- Please fill in your email correctly!
- Please describe the problem of products!
Latest Q&A


