News
The Shanghai Canbi news column provides information on reference preparations, pharmaceutical intermediates, development of pharmaceutical raw materials and blood products in the field of biomedicine. Access to the latest and most comprehensive medical news policies through various global medical news platforms, collect the latest medical research and development data, take you to understand the latest trends and policies of the pharmaceutical industry, stay at home to read the latest global medical news and information.
-
 FDA Approves AduhelmBiogen (Nasdaq: BIIB) and Eisai, Co., Ltd. (Tokyo, Japan) today announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Aduhelm™ (aducanumab-avwa) as the first and only Alzheimer’s disease treatment to address aTags:The number of readers:1515Source:2021-06-08
FDA Approves AduhelmBiogen (Nasdaq: BIIB) and Eisai, Co., Ltd. (Tokyo, Japan) today announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Aduhelm™ (aducanumab-avwa) as the first and only Alzheimer’s disease treatment to address aTags:The number of readers:1515Source:2021-06-08 -
 Incyte und MorphoSys melden Dreijahresergebnisse aus der Phase-2-Studie L-MIND zu Tafasitamab in Kombination mit Lenalidomid für die Behandlung von rezidiviertem oder refraktärem DLBCLIncyte (NASDAQ:INCY) und MorphoSys US Inc., eine hundertprozentige Tochtergesellschaft der MorphoSys AG (FSE: MOR; NASDAQ:MOR), legten heute neue Dreijahresergebnisse aus der laufenden Phase-2-Studie L-MIND zu Tafasitamab (Monjuvi®) in Kombination mit LeTags:The number of readers:1448Source:2021-06-07
Incyte und MorphoSys melden Dreijahresergebnisse aus der Phase-2-Studie L-MIND zu Tafasitamab in Kombination mit Lenalidomid für die Behandlung von rezidiviertem oder refraktärem DLBCLIncyte (NASDAQ:INCY) und MorphoSys US Inc., eine hundertprozentige Tochtergesellschaft der MorphoSys AG (FSE: MOR; NASDAQ:MOR), legten heute neue Dreijahresergebnisse aus der laufenden Phase-2-Studie L-MIND zu Tafasitamab (Monjuvi®) in Kombination mit LeTags:The number of readers:1448Source:2021-06-07 -
 Merck’s KEYTRUDA® (pembrolizumab) Given After Surgery Reduced the Risk of Disease Recurrence or Death by 32% Versus Placebo as Adjuvant Therapy in Patients With Renal Cell Carcinoma (RCMerck (NYSE: MRK), known as MSD outside the United States and Canada, today announced first-time results from the pivotal Phase 3 KEYNOTE-564 trial evaluating KEYTRUDA, Merck’s anti-PD-1 therapy, for the potential adjuvant treatment of patients with renTags:The number of readers:1632Source:2021-06-04
Merck’s KEYTRUDA® (pembrolizumab) Given After Surgery Reduced the Risk of Disease Recurrence or Death by 32% Versus Placebo as Adjuvant Therapy in Patients With Renal Cell Carcinoma (RCMerck (NYSE: MRK), known as MSD outside the United States and Canada, today announced first-time results from the pivotal Phase 3 KEYNOTE-564 trial evaluating KEYTRUDA, Merck’s anti-PD-1 therapy, for the potential adjuvant treatment of patients with renTags:The number of readers:1632Source:2021-06-04 -
 FDA Approves BrexafemmeFDA Approves Brexafemme (ibrexafungerp) as the First and Only Oral Non-Azole Treatment for Vaginal Yeast InfectionsTags:The number of readers:1640Source:2021-06-03
FDA Approves BrexafemmeFDA Approves Brexafemme (ibrexafungerp) as the First and Only Oral Non-Azole Treatment for Vaginal Yeast InfectionsTags:The number of readers:1640Source:2021-06-03 -
 Moderna Announces New Drug Substance Production Agreement with Lonza in the NetherlandsModerna, Inc. (Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that it has entered into an additional agreement with Lonza (SIX: LONN) to support drug substance manufacturing for its globalTags:The number of readers:1522Source:2021-06-02
Moderna Announces New Drug Substance Production Agreement with Lonza in the NetherlandsModerna, Inc. (Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that it has entered into an additional agreement with Lonza (SIX: LONN) to support drug substance manufacturing for its globalTags:The number of readers:1522Source:2021-06-02 -
 U.S. Food and Drug Administration Approves Bristol Myers Squibb’s Zeposia® (ozanimod), an Oral Treatment for Adults with Moderately to Severely Active Ulcerative ColitisBristol Myers Squibb (NYSE:BMY) today announced that the U.S. Food and Drug Administration (FDA) approved Zeposia® (ozanimod) 0.92 mg for the treatment of adults with moderately to severely active ulcerative colitis (UC), a chronic inflammatory bowel disTags:The number of readers:1577Source:2021-05-28
U.S. Food and Drug Administration Approves Bristol Myers Squibb’s Zeposia® (ozanimod), an Oral Treatment for Adults with Moderately to Severely Active Ulcerative ColitisBristol Myers Squibb (NYSE:BMY) today announced that the U.S. Food and Drug Administration (FDA) approved Zeposia® (ozanimod) 0.92 mg for the treatment of adults with moderately to severely active ulcerative colitis (UC), a chronic inflammatory bowel disTags:The number of readers:1577Source:2021-05-28 -
 FDA Clears AskBio’s AAV Gene Therapy for Muscular Dystrophy to Enter Phase I/IIA company known as AskBio would naturally be expected to have all the answers – and this morning, the North Carolina-based biotech announced that it had been cleared by the U.S. Food and Drug Administration (FDA) to begin testing its therapeutic candidaTags:The number of readers:1721Source:2021-05-26
FDA Clears AskBio’s AAV Gene Therapy for Muscular Dystrophy to Enter Phase I/IIA company known as AskBio would naturally be expected to have all the answers – and this morning, the North Carolina-based biotech announced that it had been cleared by the U.S. Food and Drug Administration (FDA) to begin testing its therapeutic candidaTags:The number of readers:1721Source:2021-05-26 -
 Scholar Rock Receives Fast Track Designation from the U.S. FDA for Apitegromab for the Treatment of Patients with Spinal Muscular AtrophyScholar Rock (NASDAQ: SRRK), a clinical-stage biopharmaceutical company focused on the treatment of serious diseases in which protein growth factors play a fundamental role, today announced that the U.S. Food and Drug Administration (FDA) has granted FasTags:The number of readers:1641Source:2021-05-25
Scholar Rock Receives Fast Track Designation from the U.S. FDA for Apitegromab for the Treatment of Patients with Spinal Muscular AtrophyScholar Rock (NASDAQ: SRRK), a clinical-stage biopharmaceutical company focused on the treatment of serious diseases in which protein growth factors play a fundamental role, today announced that the U.S. Food and Drug Administration (FDA) has granted FasTags:The number of readers:1641Source:2021-05-25 -
 ADC Therapeutics kündigt Vorträge beim ASCO Annual Meeting 2021 anADC Therapeutics SA (NYSE:ADCT), ein kommerziell arbeitendes Biotechnologieunternehmen, ist führend in der Entwicklung neuartiger Antikörper-Wirkstoff-Konjugate (AWK) zur Behandlung hämatologischer Malignome und solider Tumore. Das Unternehmen gab heutTags:The number of readers:1552Source:2021-05-21
ADC Therapeutics kündigt Vorträge beim ASCO Annual Meeting 2021 anADC Therapeutics SA (NYSE:ADCT), ein kommerziell arbeitendes Biotechnologieunternehmen, ist führend in der Entwicklung neuartiger Antikörper-Wirkstoff-Konjugate (AWK) zur Behandlung hämatologischer Malignome und solider Tumore. Das Unternehmen gab heutTags:The number of readers:1552Source:2021-05-21 -
 Transcenta Announced the Results of the Phase I Clinical Study of PD-L1 Antibody MSB2311 with Advanced Solid Tumors and Hematological MalignanciesTranscenta Holding Limited ("Transcenta"), a global biotherapeutics company with fully-integrated capabilities in discovery, development and manufacturing of antibody-based therapeutics, announced that the updated Phase I clinical study (NCT04272944) dataTags:The number of readers:1517Source:2021-05-20
Transcenta Announced the Results of the Phase I Clinical Study of PD-L1 Antibody MSB2311 with Advanced Solid Tumors and Hematological MalignanciesTranscenta Holding Limited ("Transcenta"), a global biotherapeutics company with fully-integrated capabilities in discovery, development and manufacturing of antibody-based therapeutics, announced that the updated Phase I clinical study (NCT04272944) dataTags:The number of readers:1517Source:2021-05-20

Featured Products
-
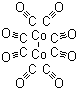 Dicobalt octacarbonylCAS:10210-68-1Molecular Formula:Co2(CO)8
Dicobalt octacarbonylCAS:10210-68-1Molecular Formula:Co2(CO)8 -
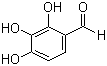 2,3,4-TrihydroxybenzaldehydeCAS:2144-08-3Molecular Formula:C7H6O4
2,3,4-TrihydroxybenzaldehydeCAS:2144-08-3Molecular Formula:C7H6O4 -
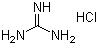 Guanidine hydrochlorideCAS:50-01-1Molecular Formula:CH5N3.HCl
Guanidine hydrochlorideCAS:50-01-1Molecular Formula:CH5N3.HCl -
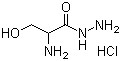 DL-Serinohydrazide monohydrochlorideCAS:55819-71-1Molecular Formula:C3H9N3O2.ClH
DL-Serinohydrazide monohydrochlorideCAS:55819-71-1Molecular Formula:C3H9N3O2.ClH -
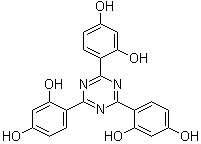 2,4,6-Tri(2,4-dihydroxyphenyl)-1,3,5-triazineCAS:2125-23-7Molecular Formula:C21H15N3O6
2,4,6-Tri(2,4-dihydroxyphenyl)-1,3,5-triazineCAS:2125-23-7Molecular Formula:C21H15N3O6 -
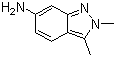 2,3-Dimethyl-6-amino-2H-indazoleCAS:444731-72-0Molecular Formula:C9H11N3
2,3-Dimethyl-6-amino-2H-indazoleCAS:444731-72-0Molecular Formula:C9H11N3 -
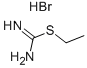 S-ETHYLISOTHIOUREA HYDROBROMIDECAS:1071-37-0Molecular Formula:
S-ETHYLISOTHIOUREA HYDROBROMIDECAS:1071-37-0Molecular Formula: -
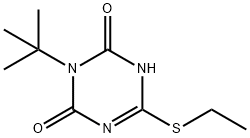 1,3,5-Triazine-2,4(1H,3H)-dione, 3-(1,1-dimethylethyl)-6-(ethylthio)-CAS:1360105-53-8Molecular Formula:
1,3,5-Triazine-2,4(1H,3H)-dione, 3-(1,1-dimethylethyl)-6-(ethylthio)-CAS:1360105-53-8Molecular Formula:
Hot Tags
Inquiry
Fill in related informations ;please!
- Please fill in product name !
- CAS
- Please fill in your company information correctly!
- Please fill in liaison information correctly!
- Please fill in your telephone number correctly!
- Please fill in your email correctly!
- Please describe the problem of products!
Latest Q&A


