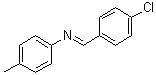
N-(4-Chlorobenzylidene)-4-methylaniline | CAS:15485-32-2
N-(4-Chlorobenzylidene)-4-methylaniline
- Name:N-(4-Chlorobenzylidene)-4-methylaniline
- CAS:15485-32-2
- Synonyms:N-(p-Chlorobenzylidene)-p-toluidine; p-Chlorobenzal-p-toluidine
- Molecular Formula:C14H12ClN
- Molecular Weight:229.70
- EINESC:
Description
Properties
| Melting point | 144 ºC** |
|---|---|
| Density | 1.07±0.1 g/cm3 (20 ºC |
Safety
| 没有数据 | 没有数据 |
|---|
SDS
| Source | SDS/MSDS Samples |
|---|---|
| 没有数据 | 没有数据 |
Synthetic Route
| Name | CAS |
|---|
More
Articles
Peer-Reviewed Papers
Quantification of DNA and hemoglobin adducts of 3,4-epoxy-1,2-butanediol in rodents exposed to 3-butene-1,2-diol.M W Powley et al.Carcinogenesis, 26(9), 1573-1580 (2005-05-13)
The importance of 3,4-epoxy-1,2-butanediol and hydroxymethylvinyl ketone in 3-butene-1,2-diol associated mutagenicity.Mark W Powley et al.Chemico-biological interactions, 166(1-3), 182-190 (2007-03-14)
An efficient, palladium-catalyzed, enantioselective synthesis of (2R)-3-butene-1,2-diol and its use in highly selective Heck reactions.Natasha Cheeseman et al.Proceedings of the National Academy of Sciences of the United States of America, 101(15), 5396-5399 (2004-02-13)
Effect of n-hexane on the disposition and toxicity of the 1,3-butadiene metabolite 3-butene-1,2-diol.Michael M Iba et al.Chemico-biological interactions, 166(1-3), 232-238 (2006-08-29)
Measurement of plasma or urinary metabolites and Hprt mutant frequencies following inhalation exposure of mice and rats to 3-butene-1,2-diol.Dale M Walker et al.Chemico-biological interactions, 166(1-3), 191-206 (2007-02-24)
Epoxide hydrolase-dependent metabolism of butadiene monoxide to 3-butene-1,2-diol in mouse, rat, and human liver.R J Krause et al.Drug metabolism and disposition: the biological fate of chemicals, 25(8), 1013-1015 (1997-08-01)
Cob(I)alamin for trapping butadiene epoxides in metabolism with rat S9 and for determining associated kinetic parameters.Hitesh V Motwani et al.Chemical research in toxicology, 22(9), 1509-1516 (2009-09-22)
First-pass metabolism of 1,3-butadiene in once-through perfused livers of rats and mice.J G Filser et al.Chemico-biological interactions, 135-136, 249-265 (2001-06-09)
Versatile and scalable synthesis of cyclic organic carbonates under organocatalytic continuous flow conditionsGerardy R, et al. Catalysis Science & Technology, 9(24), 6841-6851 (2019)
An approach based on liquid chromatography/electrospray ionization-mass spectrometry to detect diol metabolites as biomarkers of exposure to styrene and 1,3-butadiene.Shuijie Shen et al.Analytical biochemistry, 386(2), 186-193 (2008-12-30)
Synthesis and stereospecificity of 4,5-disubstituted oxazolidinone ligands binding to T-box riboswitch RNA.Crina M Orac et al.Journal of medicinal chemistry, 54(19), 6786-6795 (2011-08-05)
Mercapturic acid urinary metabolites of 3-butene-1,2-diol as in vivo evidence for the formation of hydroxymethylvinyl ketone in mice and rats.Christopher L Sprague et al.Chemical research in toxicology, 17(6), 819-826 (2004-06-23)
Development of a physiologically based toxicokinetic model for butadiene and four major metabolites in humans: global sensitivity analysis for experimental design issues.Céline Brochot et al.Chemico-biological interactions, 167(3), 168-183 (2007-04-03)
Metabolism of 3-butene-1,2-diol in B6C3F1 mice. Evidence for involvement of alcohol dehydrogenase and cytochrome p450.R A Kemper et al.Drug metabolism and disposition: the biological fate of chemicals, 26(9), 914-920 (1998-09-12)
Species and tissue differences in the toxicity of 3-butene-1,2-diol in male Sprague-Dawley rats and B6C3F1 mice.Christopher L Sprague et al.Toxicological sciences : an official journal of the Society of Toxicology, 80(1), 3-13 (2004-05-07)
Metabolism of 1,3-butadiene to toxicologically relevant metabolites in single-exposed mice and rats.Johannes Georg Filser et al.Chemico-biological interactions, 166(1-3), 93-103 (2006-04-18)
In vivo doses of butadiene epoxides as estimated from in vitro enzyme kinetics by using cob(I)alamin and measured hemoglobin adducts: an inter-species extrapolation approach.Hitesh V Motwani et al.Toxicology and applied pharmacology, 281(3), 276-284 (2014-12-03)
Detection of carboxylic acids and inhibition of hippuric acid formation in rats treated with 3-butene-1,2-diol, a major metabolite of 1,3-butadiene.Christopher L Sprague et al.Drug metabolism and disposition: the biological fate of chemicals, 31(8), 986-992 (2003-07-18)
3-Butene-1, 2-diol: An attractive precursor for the synthesis of enantiomerically pure organic compounds.Rao AVR, et al. Tetrahedron, 45(22), 7031-7040 (1989)
Gas chromatographic determination of 3-butene-1,2-diol in urine samples after 1,3-butadiene exposure.T Anttinen-Klemetti et al.Journal of chromatography. B, Biomedical sciences and applications, 730(2), 257-264 (1999-08-17)
A simplified methodology for quantitation of butadiene metabolites. Application to the study of 1,3-butadiene metabolism by rat liver microsomes.X Cheng et al.Drug metabolism and disposition: the biological fate of chemicals, 21(1), 121-124 (1993-01-01)
Hydroxymethylvinyl ketone: a reactive Michael acceptor formed by the oxidation of 3-butene-1,2-diol by cDNA-expressed human cytochrome P450s and mouse, rat, and human liver microsomes.R J Krause et al.Chemical research in toxicology, 14(12), 1590-1595 (2001-12-18)
Protection of rats against 3-butene-1,2-diol-induced hepatotoxicity and hypoglycemia by N-acetyl-l-cysteine.Christopher L Sprague et al.Toxicology and applied pharmacology, 207(3), 266-274 (2005-09-01)
On the reaction of glycerol dehydratase with but-3-ene-1,2-diol.Gregory M Sandala et al.Chemistry (Weinheim an der Bergstrasse, Germany), 15(19), 4865-4873 (2009-03-28)
Oxidation of 3-butene-1,2-diol by alcohol dehydrogenase.R A Kemper et al.Chemical research in toxicology, 9(7), 1127-1134 (1996-10-01)
Mechanism-based inactivation of coenzyme B12-dependent diol dehydratase by 3-unsaturated 1,2-diols and thioglycerol.Tetsuo Toraya et al.Journal of biochemistry, 144(4), 437-446 (2008-07-01)
Hepatic microsomal metabolism of 1,3-butadiene.E Malvoisin et al.Xenobiotica; the fate of foreign compounds in biological systems, 12(2), 137-144 (1982-02-01)
But-3-ene-1,2-diol: a mechanism-based active site inhibitor for coenzyme B12-dependent glycerol dehydratase.Antonio J Pierik et al.Chembiochem : a European journal of chemical biology, 9(14), 2268-2275 (2008-09-04)
Remarks
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| Name | CAS |
|---|

Related Products
Related News
Question
Hot Tags
Inquiry
Fill in related informations ;please!
- Please fill in product name !
- CAS
- Please fill in your company information correctly!
- Please fill in liaison information correctly!
- Please fill in your telephone number correctly!
- Please fill in your email correctly!
- Please describe the problem of products!