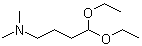
4,4-Diethoxy-N,N-dimethyl-1-butanamine | CAS:1116-77-4
4,4-Diethoxy-N,N-dimethyl-1-butanamine
- Name:4,4-Diethoxy-N,N-dimethyl-1-butanamine
- CAS:1116-77-4
- Synonyms:N,N-Dimethyl-4-aminobutanal diethyl acetal
- Molecular Formula:C10H23NO2
- Molecular Weight:189.30
- EINESC:
Description
Properties
| Flash point | 158 ºF |
|---|---|
| Density | 0.884 |
| Refractive index | 1.421 |
Safety
| Symbol(GHS) | Xi |
|---|---|
| Hazard Codes | R41 |
| SDS/MSDS | S26;S36/37/39 |
SDS
| Source | SDS/MSDS Samples |
|---|---|
| Alfa-Aesar | View & Download |
| Sigma-Aldrich | View & Download |
| TCI | View & Download |
Synthetic Route
RelatedAPI's
| Name | CAS | |
|---|---|---|
| Sumatriptan | 103628-46-2 | View |
| Sumatriptan succinate | 103628-48-4 | View |
| Zolmitriptan | 139264-17-8 | View |
More
Articles
Peer-Reviewed Papers
Discovery and optimization of imidazo[1,2-a]pyridine inhibitors of insulin-like growth factor-1 receptor (IGF-1R).Kyle A Emmitte et al.Bioorganic & medicinal chemistry letters, 19(3), 1004-1008 (2008-12-23)
Orally active C-6 heteroaryl- and heterocyclyl-substituted imidazo[1,2-a]pyridine acid pump antagonists (APAs).Nick Bailey et al.Bioorganic & medicinal chemistry letters, 19(13), 3602-3606 (2009-05-27)
One-pot three-component synthesis of 3-nitro-2-arylimidazo[1,2-a]pyridine derivatives using air as an oxidant.Hao Yan et al.Chemistry, an Asian journal, 7(9), 2028-2031 (2012-06-14)
Conversion of pyridine to imidazo[1,2-a]pyridines by copper-catalyzed aerobic dehydrogenative cyclization with oxime esters.Huawen Huang et al.Organic letters, 15(24), 6254-6257 (2013-11-23)
A novel series of imidazo[1,2-a]pyridine derivatives as HIF-1alpha prolyl hydroxylase inhibitors.Namal C Warshakoon et al.Bioorganic & medicinal chemistry letters, 16(21), 5598-5601 (2006-09-12)
Identification of novel imidazo[1,2-a]pyridine inhibitors targeting M. tuberculosis QcrB.Katherine A Abrahams et al.PloS one, 7(12), e52951-e52951 (2013-01-10)
Imidazo[1,2-a]pyridines susceptible to excited state intramolecular proton transfer: one-pot synthesis via an Ortoleva-King reaction.Anton J Stasyuk et al.The Journal of organic chemistry, 77(13), 5552-5558 (2012-06-06)
Fused heterocycles: synthesis of some new imidazo[1,2-a]- pyridine derivatives.Birgul Ozden Kasimogullari et al.Molecules (Basel, Switzerland), 9(10), 894-901 (2007-11-17)
Functionalized heterocyclic scaffolds derived from Morita-Baylis-Hillman Acetates.Huajian Zhu et al.Chemical communications (Cambridge, England), 49(70), 7738-7740 (2013-07-24)
Design, synthesis and in vitro antimicrobial evaluation of novel Imidazo[1,2-a]pyridine and imidazo[2,1-b][1,3]benzothiazole motifs.Taleb H Al-Tel et al.European journal of medicinal chemistry, 46(5), 1874-1881 (2011-03-19)
Molecular characterization of EP6--a novel imidazo[1,2-a]pyridine based direct 5-lipoxygenase inhibitor.Joanna M Wisniewska et al.Biochemical pharmacology, 83(2), 228-240 (2011-10-27)
Intra-periaqueductal grey microinjections of an imidazo[1,2-b]pyridazine derivative, DM2, affects rostral ventromedial medulla cell activity and shows antinociceptive effect.Enza Palazzo et al.Neuropharmacology, 58(3), 660-667 (2009-12-01)
SAR-study on a new class of imidazo[1,2-a]pyridine-based inhibitors of 5-lipoxygenase.Martina Hieke et al.Bioorganic & medicinal chemistry letters, 22(5), 1969-1975 (2012-02-14)
Colorimetric determination of cysteine by a paper-based assay system using aspartic acid modified gold nanoparticles.Chunye Liu et al.Mikrochimica acta, 187(6), 362-362 (2020-06-02)
Design and biological evaluation of imidazo[1,2-a]pyridines as novel and potent ASK1 inhibitors.Yoshito Terao et al.Bioorganic & medicinal chemistry letters, 22(24), 7326-7329 (2012-11-14)
Structural insight for imidazopyridazines as malarial kinase PfPK7 inhibitors using QSAR techniques.Nitendra K Sahu et al.Medicinal chemistry (Shariqah (United Arab Emirates)), 8(4), 636-648 (2012-04-26)
Post Groebke-Blackburn multicomponent protocol: synthesis of new polyfunctional imidazo[1,2-a]pyridine and imidazo[1,2-a]pyrimidine derivatives as potential antimicrobial agents.Taleb H Al-Tel et al.European journal of medicinal chemistry, 45(12), 5848-5855 (2010-10-12)
Synthesis and biological evaluation of 2,3-diarylimidazo[1,2-a]pyridines as antileishmanial agents.Sophie Marhadour et al.European journal of medicinal chemistry, 58, 543-556 (2012-11-21)
Imidazo[1,2-a]pyridine derivatives as inhibitors of TNF-alpha expression in T cells.Jan Rether et al.Bioorganic & medicinal chemistry, 16(3), 1236-1241 (2007-11-24)
Discovery of novel imidazo[1,2-a]pyridines as inhibitors of the insulin-like growth factor-1 receptor tyrosine kinase.Richard Ducray et al.Bioorganic & medicinal chemistry letters, 21(16), 4698-4701 (2011-07-22)
Novel one-pot three component reaction for the synthesis of [2-(alkylsulfanyl)imidazo[1,2-a]pyridin-3-yl](aryl)methanone.Ebrahim Kianmehr et al.Journal of combinatorial chemistry, 12(1), 41-44 (2009-11-13)
IRAK-4 inhibitors. Part III: a series of imidazo[1,2-a]pyridines.George M Buckley et al.Bioorganic & medicinal chemistry letters, 18(12), 3656-3660 (2008-05-27)
Generation and exploration of new classes of antitubercular agents: The optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds.Garrett C Moraski et al.Bioorganic & medicinal chemistry, 20(7), 2214-2220 (2012-03-07)
Three-component, one-pot sequential synthesis of functionalized cyclazines: 3H-1,2a1,3-triazaacenaphthylenes.Hongpeng Sun et al.The Journal of organic chemistry, 77(23), 10745-10751 (2012-11-22)
Synthesis and antiprotozoal activity of novel bis-benzamidino imidazo[1,2-a]pyridines and 5,6,7,8-tetrahydro-imidazo[1,2-a]pyridines.Mohamed A Ismail et al.Bioorganic & medicinal chemistry, 16(2), 683-691 (2007-11-03)
A base promoted cyclization of N-propargylaminopyridines. Synthesis of imidazo[1,2-a]pyridine derivatives.Suren Husinec et al.Organic letters, 13(9), 2286-2289 (2011-03-31)
Synthesis and biological evaluation of imidazo[1,2-a]pyridine derivatives as novel PI3 kinase p110alpha inhibitors.Masahiko Hayakawa et al.Bioorganic & medicinal chemistry, 15(1), 403-412 (2006-10-20)
Novel imidazo[1,2-a]pyridine based inhibitors of the IGF-1 receptor tyrosine kinase: optimization of the aniline.Richard Ducray et al.Bioorganic & medicinal chemistry letters, 21(16), 4702-4704 (2011-07-19)
Efficient solid-phase synthesis of a library of imidazo[1,2-a]pyridine-8-carboxamides.Ahmed Kamal et al.Journal of combinatorial chemistry, 9(2), 267-274 (2007-02-27)
Remarks
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).

Related Products
Related News
Question
Hot Tags
Inquiry
Fill in related informations ;please!
- Please fill in product name !
- CAS
- Please fill in your company information correctly!
- Please fill in liaison information correctly!
- Please fill in your telephone number correctly!
- Please fill in your email correctly!
- Please describe the problem of products!