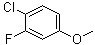
4-Chloro-3-fluoroanisole | CAS:501-29-1
4-Chloro-3-fluoroanisole
- Name:4-Chloro-3-fluoroanisole
- CAS:501-29-1
- Synonyms:
- Molecular Formula:C7H6ClFO
- Molecular Weight:160.57
- EINESC:
Description
Properties
| Boiling point | 176.9±20.0 ºC (760 Tor |
|---|---|
| Flash point | 60.8±21.8 ºC, 计算值* |
| Density | 1.239±0.06 g/cm3 (20 º |
Safety
| 没有数据 | 没有数据 |
|---|
SDS
| Source | SDS/MSDS Samples |
|---|---|
| 没有数据 | 没有数据 |
Synthetic Route
| Name | CAS |
|---|
More
Articles
Peer-Reviewed Papers
Allergic contact dermatitis with spreading over extensive regions due to topical use of 5% bufexamac ointment.Junko Arikawa et al.The Journal of dermatology, 31(2), 136-138 (2004-05-27)
Contact sensitivity to bufexamac.Lachapelle JM. Contact Dermatitis, 1(4), 261-261 (1975)
Examinations of the antioxidative properties of the topically administered drug bufexamac reveal new insights into its mechanism of action.Hagen Trommer et al.The Journal of pharmacy and pharmacology, 55(10), 1379-1388 (2003-11-11)
Identification of clinically approved small molecules that inhibit growth and affect transcript levels of developmentally regulated genes in the African trypanosome.Madison Elle Walsh et al.PLoS neglected tropical diseases, 14(3), e0007790-e0007790 (2020-03-14)
Chronic urticaria due to Blastocystis hominis.Ritu Gupta et al.The Australasian journal of dermatology, 47(2), 117-119 (2006-04-28)
Effects of intra-articular injections of bufexamac suspension on amphotericin B-induced aseptic arthritis in horses.M M Suominen et al.American journal of veterinary research, 60(12), 1467-1473 (2000-01-06)
EMA revokes marketing authorization for bufexamac.Wolfgang Uter et al.Contact dermatitis, 64(4), 235-236 (2011-03-12)
[Bufexamac-induced pigmented purpuric eruption].K Waltermann et al.Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete, 60(5), 424-427 (2008-12-19)
Delayed contact hypersensitivity to non-steroidal anti-inflammatory drugs.B Gniazdowska et al.Contact dermatitis, 40(2), 63-65 (1999-02-27)
An integrative approach to eczema (atopic dermatitis).Stephanie Maxine Ross Holistic nursing practice, 17(1), 56-62 (2003-02-25)
Allergic contact dermatitis to topical preparations of bufexamac.Yan Pan et al.The Australasian journal of dermatology, 53(3), 207-210 (2012-08-14)
In vitro photobiochemical characterization of sulfobutylether-β-cyclodextrin formulation of bufexamac.Yoshiki Seto et al.Journal of pharmaceutical and biomedical analysis, 55(3), 591-596 (2011-03-25)
Effect of nadifloxacin on atopic dermatitis with methicillin-resistant Staphylococcus aureus in young children.H Kimata European journal of pediatrics, 158(11), 949-949 (1999-12-14)
Changes in MMP-2 and -9 activity and MMP-8 reactivity after amphotericin B induced synovitis and treatment with bufexamac.P H Marttinen et al.Journal of veterinary medicine. A, Physiology, pathology, clinical medicine, 53(6), 311-318 (2006-08-12)
Erythema-multiforme-like, urticarial papular and plaque eruptions from bufexamac: report of 4 cases.P Koch et al.Contact dermatitis, 31(2), 97-101 (1994-08-01)
A systematic review of randomized controlled trials of treatments for inherited forms of epidermolysis bullosa.S M Langan et al.Clinical and experimental dermatology, 34(1), 20-25 (2008-10-03)
[Severe allergic contact dermatitis with generalized spread due to bufexamac presenting as the "baboon" syndrome].S Proske et al.Deutsche medizinische Wochenschrift (1946), 128(11), 545-547 (2003-03-14)
Allergic contact dermatitis syndrome from bufexamac for nursing infant.Tokio Nakada et al.Dermatitis : contact, atopic, occupational, drug, 23(4), 185-186 (2012-07-26)
Effects of intra-articular injections of bufexamac suspension in healthy horses.M M Suominen et al.American journal of veterinary research, 62(10), 1629-1635 (2001-10-11)
Selective enhancement of production of IgE, IgG4, and Th2-cell cytokine during the rebound phenomenon in atopic dermatitis and prevention by suplatast tosilate.H Kimata Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology, 82(3), 293-295 (1999-03-27)
[A common and insidious side-effect: allergic contact dermatitis caused by bufexamac used in the treatment of dermatitis. Results from the Information Network of Departments of Dermatology (IDVK)].A Schnuch et al.Deutsche medizinische Wochenschrift (1946), 130(50), 2881-2886 (2005-12-13)
Determination of bufexamac in cream and ointment by high-performance liquid chromatography.Kamata K, et al. Journal of Chromatography A, 370(3), 344-347 (1986)
[Mastu S - a new antihemorrhoidal cream for pregnant women].L Damjanov Akusherstvo i ginekologiia, 41 Suppl 2, 26-27 (2002-01-01)
[Anogenital dermatoses--allergic and irritative causative factors. Analysis of IVDK data and review of the literature].Katrin Kügler et al.Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG, 3(12), 979-986 (2006-01-13)
Bufexamac and diclofenac: frequency of contact sensitization and absence of cross-reactions.A Barbaud et al.Contact dermatitis, 39(5), 272-273 (1998-12-05)
Non-steroidal anti-inflammatory drugs selectively inhibit cytokine production by NK cells and gamma delta T cells.Miyuki Inaoka et al.Experimental dermatology, 15(12), 981-990 (2006-11-07)
[A severe epicutaneous test reaction to the bufexamac in a hemorrhoidal therapeutic preparation].A Bauer et al.Deutsche medizinische Wochenschrift (1946), 124(40), 1168-1170 (1999-11-05)
Contact dermatitis caused by bufexamac sparing the eruption of herpes zoster.Hidetsugu Fukuda et al.The Journal of dermatology, 39(4), 405-407 (2011-09-29)
Acute generalized exanthematous pustulosis induced by bufexamac in an atopic girl.H Belhadjali et al.Contact dermatitis, 58(4), 247-248 (2008-03-21)
Efficacy of bufexamac (NFN) cream in skin diseases.VJC, et al. Dermatology (Basel, Switzerland), 154(3), 177-184 (1977)
Remarks
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer's risk.
Products currently covered by valid US Patents are offered for R&D use in accordance with 35 USC 271 +A13(1).
| Name | CAS |
|---|

Related Products
Related News
Question
Hot Tags
Inquiry
Fill in related informations ;please!
- Please fill in product name !
- CAS
- Please fill in your company information correctly!
- Please fill in liaison information correctly!
- Please fill in your telephone number correctly!
- Please fill in your email correctly!
- Please describe the problem of products!